Research Focus on Prof. Chuanbing Tang
College of Arts and Sciences Distinguished Professor
Department of Chemistry and Biochemistry
University of South Carolina
Dr. Chuanbing Tang is a Distinguished Professor in the Department of Chemistry and Biochemistry at the University of South Carolina. He is a Contributing Faculty in the NSF MADE in SC Track-1 project for Thrust 2 and Thrust 3. Dr. Tang was recently elected as a 2020 Fellow of AAAS. The following are research projects that represent a sample of his contribution to MADE in SC.
Mechano-Responsive Polymers (Thrust 2)
Stimuli-responsive polymers have vast applications in a variety of areas. Among them, mechano-responsive polymers demonstrate specific responses to an external stress. These polymers are usually centered around mechanophores, a class of stress-responsive entities, which are connected to the polymer or polymer matrix such that stored tension is coupled to a specific chemical response. Recently, we discovered that ferrocene mechanophores embedded in polymer backbones can be mechanically activated under sonication (Figure 1). We further quantified the mechanical strength of ferrocene, and found that it displays remarkable mechanochemical lability despite its substantial thermal bond dissociation energy.
This project adopts an MGI approach to combining density functional theory calculation with experimental design. The experimental part is focused on the synthesis of mechano-responsive main-chain metallocene-containing polymers. The simulation efforts include constrained geometries simulated external force (COGEF) potential and force, structural evolution and electrostatic potential map of mechanophore compounds. The DFT calculation can predict bond dissociation energy and force to break metal-ligand bond in metallocenes. The COGEF simulations provide more mechanistic insights into the mechanochemical process of mechanophore dissociation to see how the interplay of bond length and bond strength plays out. Experimentally, we combine ring-closing metathesis (RCM) and ring-opening metathesis polymerization (ROMP) to prepare main-chain mechanophore-containing polymers. RCM allows the synthesis of various cyclic olefin monomers. Entropy-driven ring-opening metathesis polymerization (ED-ROMP) is used to synthesize polymers of varying molecular weight and mechanophore content. One of challenging tasks is how to trap and characterize dissociated structures, which are critical to understand the mechanism of chain scission during mechanical stress under ultrasonication.

Figure 1. Mechano-responsive polymers using metallocenes as a new class of mechanophores that exhibit an intriguing combination of force-free thermal stability and mechanical lability.
Reference: Sha Y.; Zhang Y.; Xu E.; McAlister W.; Zhu T.; Craig S. L.; Tang, C. Generalizing Metallocene Mechanochemistry to Ruthenocene Mechanophores. Chem. Sci. 2019, 10, 4959-4965.
Antimicrobial Polymers (Thrust 3)
Bacterial infections and antimicrobial resistance have become a global healthcare crisis. Antimicrobial peptides (AMPs) are the first line of host defense system. Upon contact with bacterial cell membranes, many AMPs adopt a facial amphiphilic conformation with one side carrying hydrophilic positive charges and the other side having lipophilic groups (Figure 2a). This conformation is made possible, as AMPs possess an α-helix structure. AMP-mimicking polymers typically also contain hydrophilic charges and hydrophobic moieties, which are targeted at a global amphiphilicity (Figure 2b). However, most of these polymers rely on uncontrolled polymeric self-aggregation, which would overcome significant entropy loss if the polymers want to form a facial amphiphilicity. We develop a class of cationic cholic acid-based polymers that possess local facial amphiphilicity clustered together via a flexible macromolecular chain (Figure 2c). Cationic quaternary ammonium charges (QAC) are derived from the hydroxyl group-containing α-face in cholic acid while the β-face of multicyclic hydrocarbon rings is intrinsically hydrophobic.
This unique macromolecular composition overcomes many of the deficiencies of other antimicrobial peptides and antimicrobial polymers. First, it adopts the facial amphiphilicity similar to host defense peptides, but not necessarily possessing a helical conformation; Second, it does not need to overcome the near-impossible changes in global conformational arrangements that most previous antimicrobial polymers have required to adapt to make facial amphiphilicity with large entropic loss. This paves the way to build a new platform of next-generation antimicrobial biomaterials that have greatly reduced potential for resistance development and cytotoxic effects. The work will push the field ahead in dealing with antimicrobial resistance. This project involves molecular dynamic simulation. The experimental part is focused on synthesis of different antimicrobial polymers and antimicrobial tests. The simulation efforts include: (1) to understand the interaction with bacterial cell membranes; (2) to provide feedback to design better compositions.
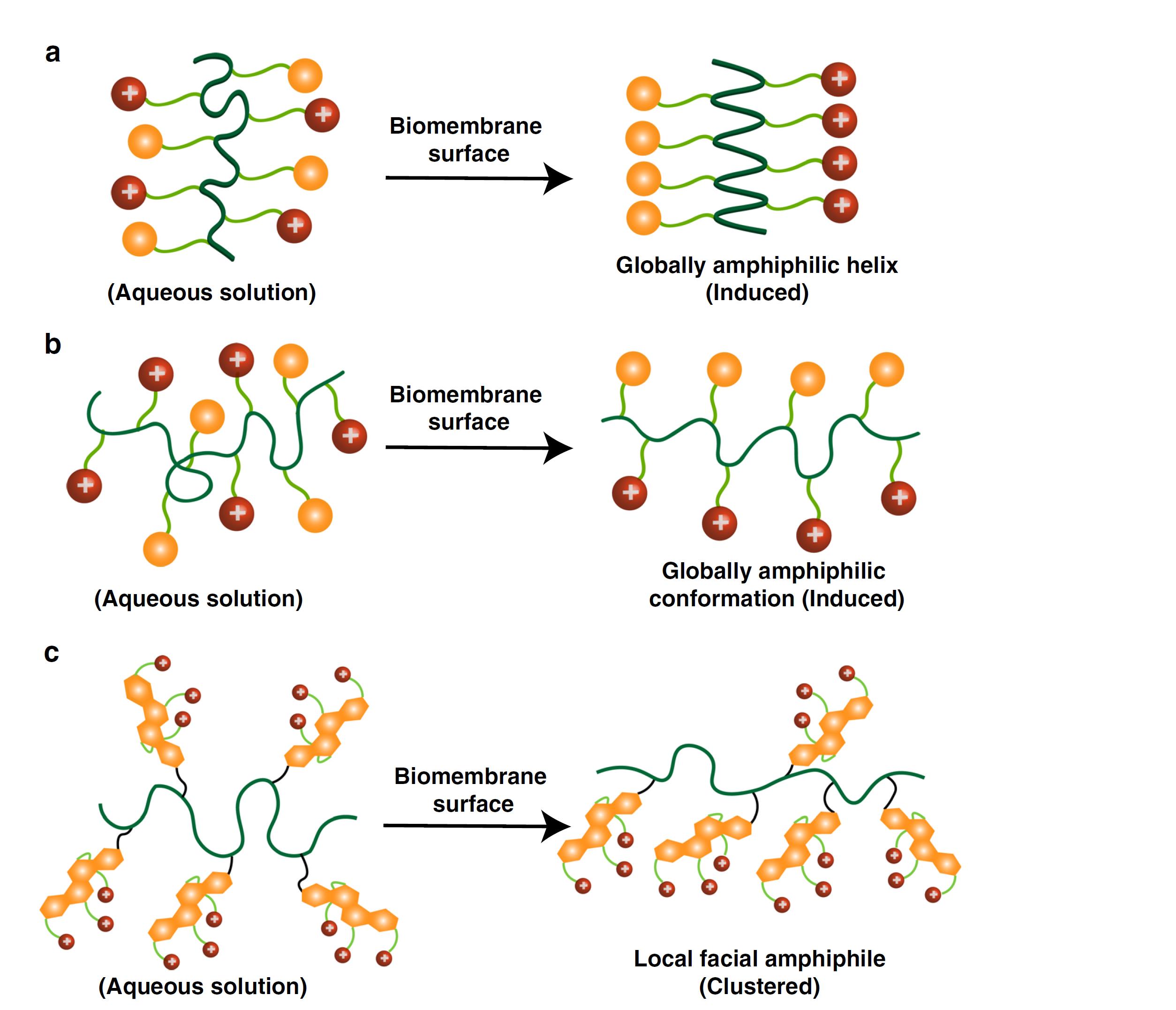
Figure 2. Mechanisms of action of antimicrobial macromolecules upon contact with biomembrane: a. antimicrobial peptides with a helical facial amphiphilic conformation; b. antimicrobial polymers having a globally amphiphilic conformation; c. a flexible macromolecular chain with local facial amphiphiles at each repeat unit.
Reference: Rahman M. A.; Bam M.; Luat E.; Jui M. S.; Ganewatta M.; Shokfai T.; Nagarkatti M.; Decho A.; Tang C. Macromolecular-Clustered Facial Amphiphilic Antimicrobials. Nat. Commun. 2018, 9, 5231.
December 2020
