Intersection of Epigenetic Regulation and Mitochondrial Function in Autism
Jeff Twiss, Professor, Interim Departmental Chair, SmartState Chair in Childhood Neurotherapeutics, Department of Biological Sciences, University of South Carolina - PI
A Stimulus Research Program Award Supported by the SC EPSCoR Program
(SC EPSCoR SRP Award 18-SR04)
The Research Team:
- Jeff Twiss, Professor, Interim Departmental Chair, SmartState Chair in Childhood Neurotherapeutics, Department of Biological Sciences, University of South Carolina
- Sofia Lizarraga, Assistant Professor, Department of Biological Sciences, University of South Carolina
- Kevin Champaigne, Research Assistant Professor, Department of Bioengineering, Clemson University
- Linnea Freeman, Assistant Professor, Department of Biology, Furman University
- Luigi Boccuto, Clinical Lecturer, School of Nursing, Clemson University
- Omar Bagasra, Professor, Department of Biology, Claflin University
Approximately 1 in 59 individuals in the United States have Autism Spectrum Disorder and this may even be more frequent in South Carolina. Autism manifests as a spectrum of symptoms that include difficulties with social interactions, verbal and nonverbal communication, and restricted/repetitive behaviors. Unfortunately, there is no single event that precipitates autism, with many different gene mutations as well as environmental exposures increasing the risk for a child to develop autism. Several lines of evidence point to disruption of neural connections in the brains of children with autism, and similar is seen in rodent models of autism spectrum disorder. There is no magic ‘pill’ for treating autism, so behavioral interventions remain the mainstay of treatment. Those interventions take advantage of the natural ‘plasticity’ of the brain to modify connections between neurons. This SC Stimulus Award focuses on understanding the molecular mechanisms underlying those plastic events.
Our collaboration precipitated from a grass roots effort of researchers across SC’s academic and research institutions that formed the South Carolina Autism and Neurodevelopmental Disorders (SCAND) Consortium in 2016. Our Stimulus Award project includes Omar Bagasra (Claflin Univ.), Luigi Boccuto (Greenwood Genetics Ctr. [now at Clemson Univ.]), Kevin Champaigne (Clemson Univ.), Linnea Freeman (Furman Univ.), and Sofia Lizarraga (Univ. South Carolina) as co-PIs with Jeff Twiss (Univ. South Carolina) as lead PI. We built upon metabolomics data from Boccuto and colleagues pointing to altered utilization of tryptophan in cells from children with autism compared those from typically developing children (ref) and emerging molecular data from Lizarraga pointing to altered mitochondrial function in human neurons treated with the autism risk factor valproic acid [VPA]. VPA is an anti-epileptic but it also alters epigenetic regulation of gene expression, and offspring of mothers receiving this drug during pregnancy are at a greater risk of developing autism. Tryptophan is a precursor of NAD that is needed for mitochondrial function, so we surmised that mitochondrial function may be uniquely impaired in autism – Our team set out to test this hypothesis with the Stimulus Award.
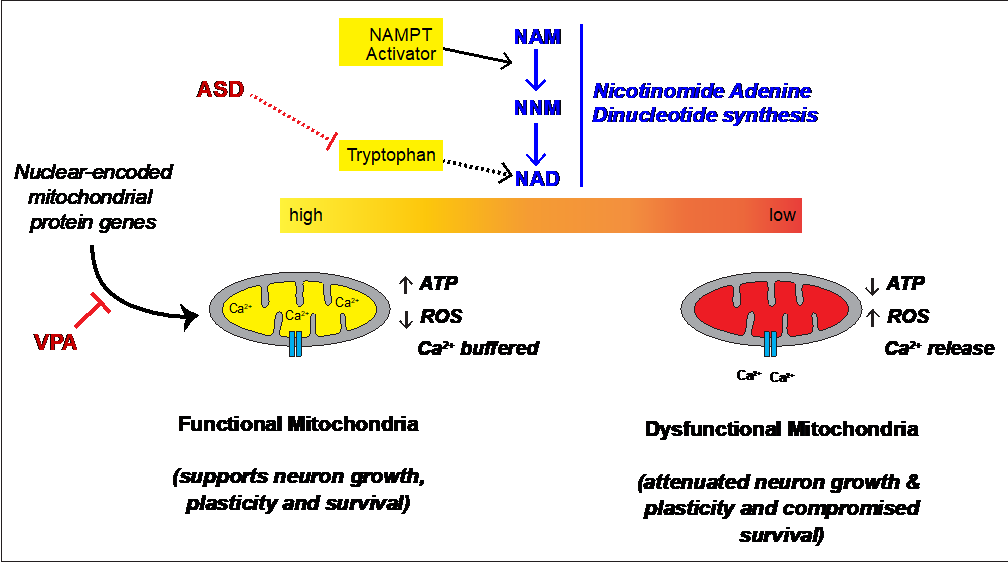
We took a complimentary multi-pronged approach to test our hypothesis across human cell lines, mouse neurons, human neurons and mice focusing on the environmental agents that are linked to increased prevalence of autism. We uncovered surprising differences in male vs. female neurons and lymphoblasts that spans across mouse and human. Specifically, we see a striking difference in mitochondrial membrane potential between male and female neurons harvested directly from normal embryonic mice. Moreover, the male neurons show an exaggerated response to VPA. Lizarraga’s lab found that stem cell-derived human male and female neurons have different synaptic activity, with the male neurons again showing an exaggerated response to VPA. Boccuto and Champaigne further found that VPA treatment of lymphoblasts from typically developing children have metabolomic profiles paralleling lymphoblasts from autistic children. Critically, they were able to revert these profiles using an activator of NAD synthesis. Together, these findings indicate that the autism susceptibility agent VPA alters mitochondrial function in humans and mice and in multiple cell types. Mitochondrial function is needed for growth of neurons and formation of synapses, and we suspect that VPA modulation of gene expression is at the crux of these changes (Figure 1). Consistent with this, VPA-treated mouse neurons show altered expression genes encoding mitochondrial proteins similar to the human neurons. Bagasra further showed that other compounds linked to autism susceptibility can differentially alter gene expression in human male vs. female neuroblastoma cell lines.
 |
|
Fig 1 – Schematic for proposed role of mitochondrial respiration in autism. Previous work from Boccuto and colleagues point to altered tryptophan utilization in ASD. Based on work in mouse and human neurons and human lymphoblasts, we see that the ASD susceptibility agent VPA decreases mitochondrial function, alters neuronal function, and disrupted expression of proteins needed for mitochondrial function. Exogenous activation of NAMPT reverses the VPA effects in lymphoblasts. This provides new insight into the pathophysiology of autism. |
An essential component of this collaboration is the ability to modify the directions in each lab based on the sum of data emerging from the group, both for testing our hypothesis and refining our experimental approach and questions. Based on a higher prevalence of autism in males compared to female children, we anticipated finding differences in males and females. But we thought this would be at the behavioral rather than cellular level as outlined above. Freeman developed a ‘first in SC’ in utero autism model with VPA exposure to test the behaviorally outcomes of these differences and explore the role of mitochondrial function in this. The cellular findings from across our labs have been used to guide those mouse studies, and the mouse model will serve as a platform to test new hypothesis and new interventions that we will leverage for continued funding for this collaborative research.
Beyond the scientific advances outlined above, our collaborative approach to research brought new opportunities for cross-mentoring students and fellows in neurodevelopmental disorders research. This brought undergraduate students from Furman Univ. new opportunities for exposure to molecular neurobiology work at UofSC and students from Claflin Univ. new opportunities for exposure to animal research at Furman Univ. (Figure 2). One of those Claflin students is now pursuing their Ph.D. at the Univ. Arkansas. Other students have gone off to graduate and medical schools. This project also brought opportunities to mentor junior faculty, such as broadening the neurobiology expertise that Freeman could draw from in preparing her independent grant proposals. Finally, this cross institution work encouraged the investigators to follow new leads beyond the technical feasibilities of their institutions by facilitating access to instrumentation and expertise across SC.
 |
|
|
August 2020